PREVIOUS
ONDLS for drug license
October 12 , 2025
13 hrs 0 min
6
0
- 18 State drug control authorities have adopted the Online National Drugs Licensing System (ONDLS) for drug license processing.
- No State has fully complied with the Corrective and Preventive Action (CAPA) guidelines.
- Both ONDLS and CAPA are provisions under the Central government’s revised Schedule M, which updates India’s pharmaceutical manufacturing regulations.
- CAPA is a quality management method that focuses on investigating and preventing problems in drug production.
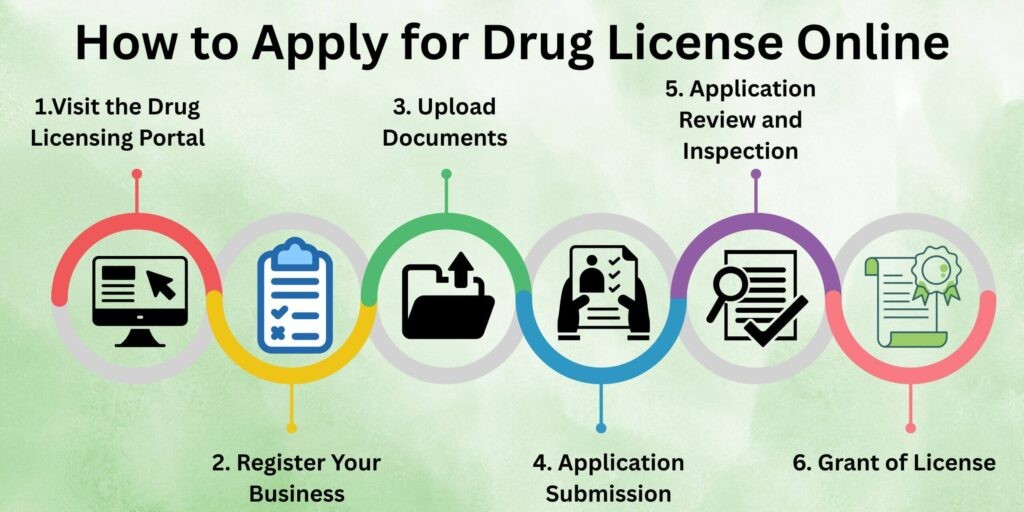
- The ONDLS is a digital single-window platform designed to streamline and standardize drug licensing across all States and Union Territories.
- Out of 5,308 MSME pharma companies, 3,838 have complied with the revised Schedule M Good Manufacturing Practices (GMP).
Leave a Reply
Your Comment is awaiting moderation.


